A sampling of ongoing research in the lab….
Theme I: functions of the nuclear lamina
How do the three lamin isoforms – lamin A/C, lamin B1, and lamin B2 – each contribute to nuclear function?
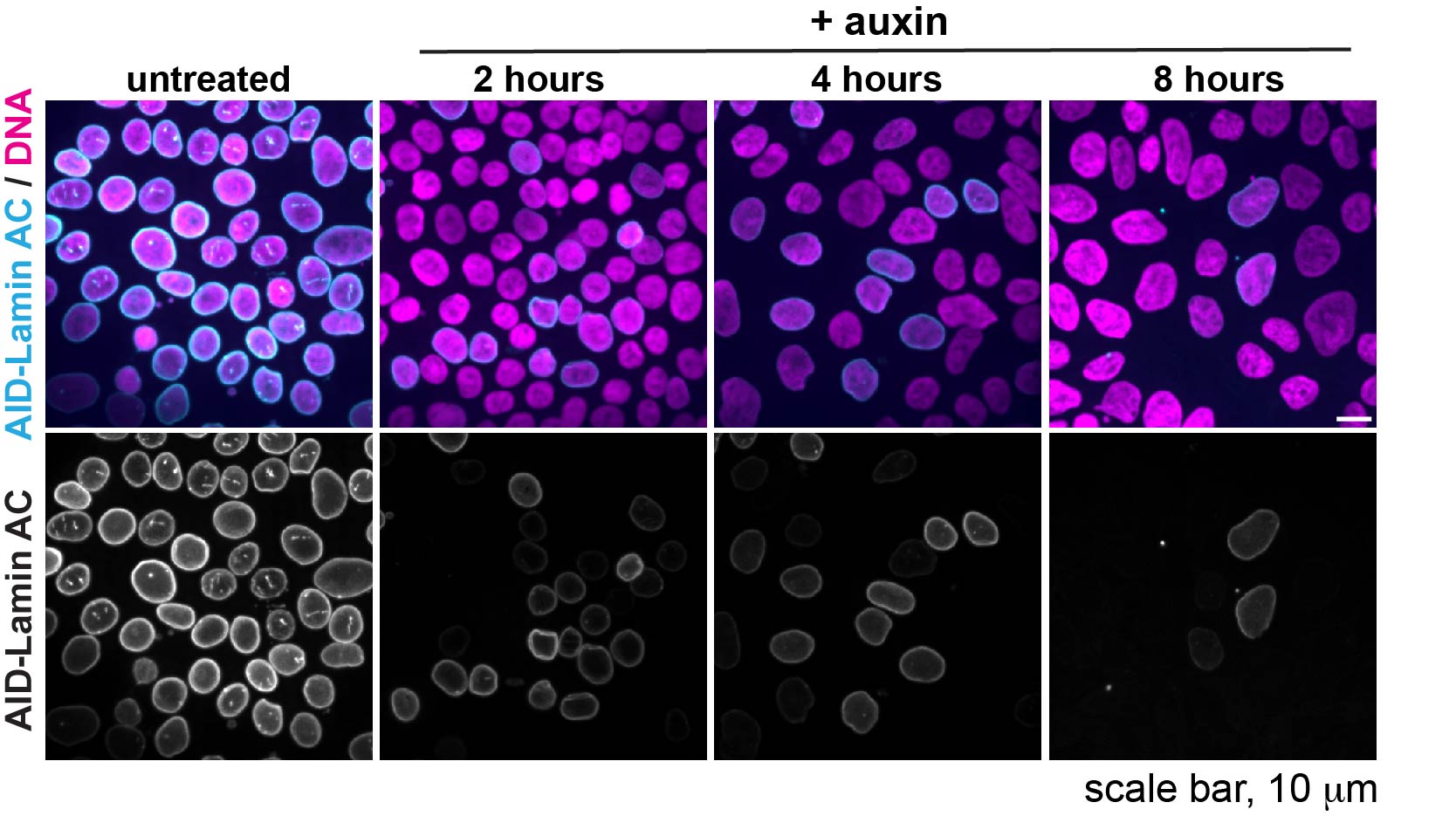
We are using auxin-inducible degrons to acutely deplete each lamin isoform within one cell cycle and assess the immediate consequences on nuclear organization and gene expression.
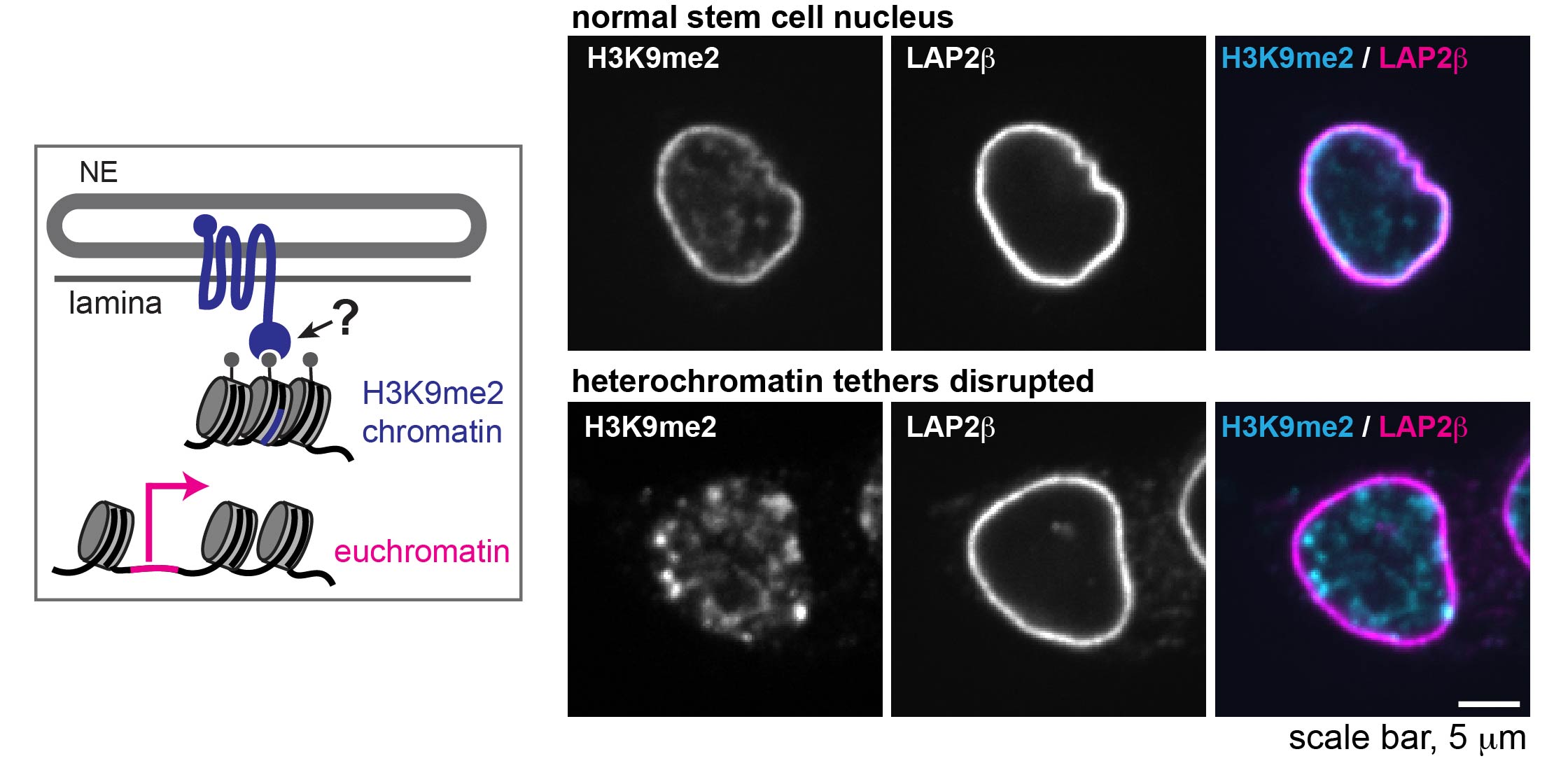
How is heterochromatin tethered to the lamina, and why does it matter?
We are using embryonic stem cell differentiation and gastruloids to explore the importance of heterochromatin tethering for regulation of gene expression and establishment of cell fate in early development.
Theme II: dynamic regulation of the nuclear lamina
How is the nuclear lamina remodeled?
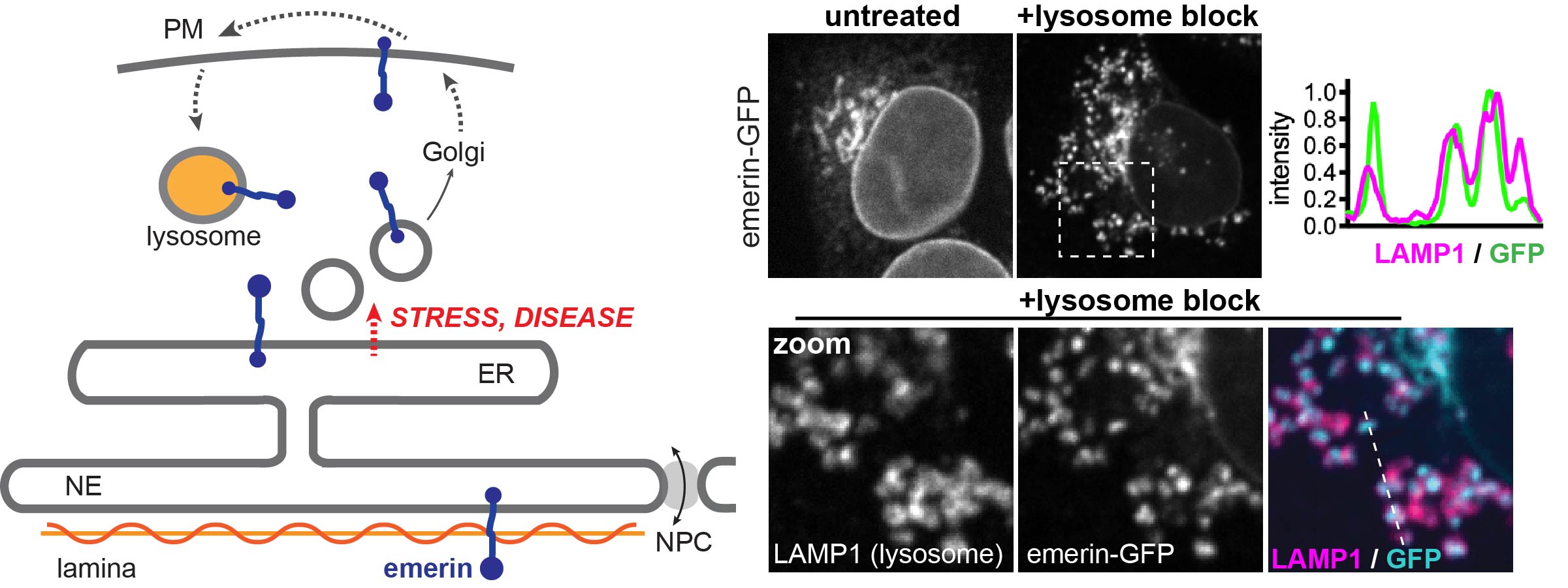
Proteins of the nuclear envelope membrane are dual citizens: they function in the nucleus but are made in the endoplasmic reticulum, which is part of the secretory pathway. We discovered that emerin, a lamin-binding membrane protein, can be sent either to the nucleus or through the secretory pathway to the plasma membrane, and finally to the lysosome. We are dissecting the mechanism of this unusual targeting pathway and testing whether it enables a novel non-nuclear function for emerin or alternatively antagonizes the protein’s function by promoting its degradation.
See previous publication here.
How do post-translational modifications regulate the lamina?
***under construction***
Theme III: nuclear dysfunction in disease
Why do mutations to the lamin proteins cause tissue-specific “laminopathy” diseases?
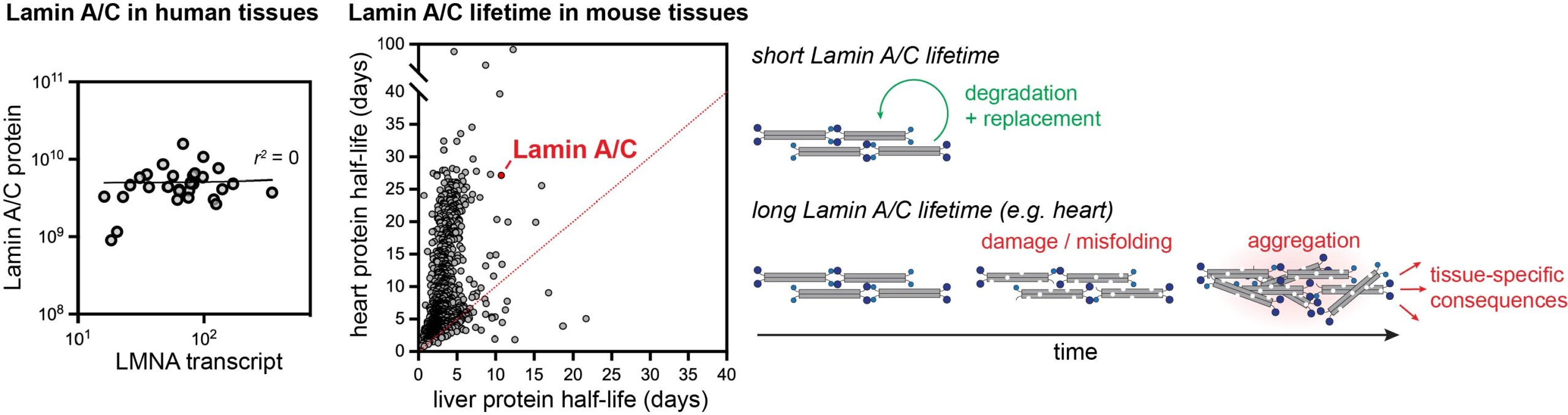
Mutations to the LMNA gene, which encodes lamin A/C, cause cardiomyopathy, muscular dystrophy, lipodystrophy, neuropathy, and other rarer syndromes such as Hutchinson-Gilford progeria syndrome. We are curious how mutations to a broadly expressed protein can cause such diverse and tissue-specific phenotypes. We discovered that the lamin A/C proteins are unusually long-lived in the tissues that are most vulnerable to laminopathy diseases, such as the heart. We are exploring how variation in protein lifetime alters the functions of the lamin proteins across tissues, and how disease mutations influence protein stability and protein function across tissues.
See publication here.
Theme IV: nuclear proteostasis
Building new tools to profile nuclear proteostasis in vivo
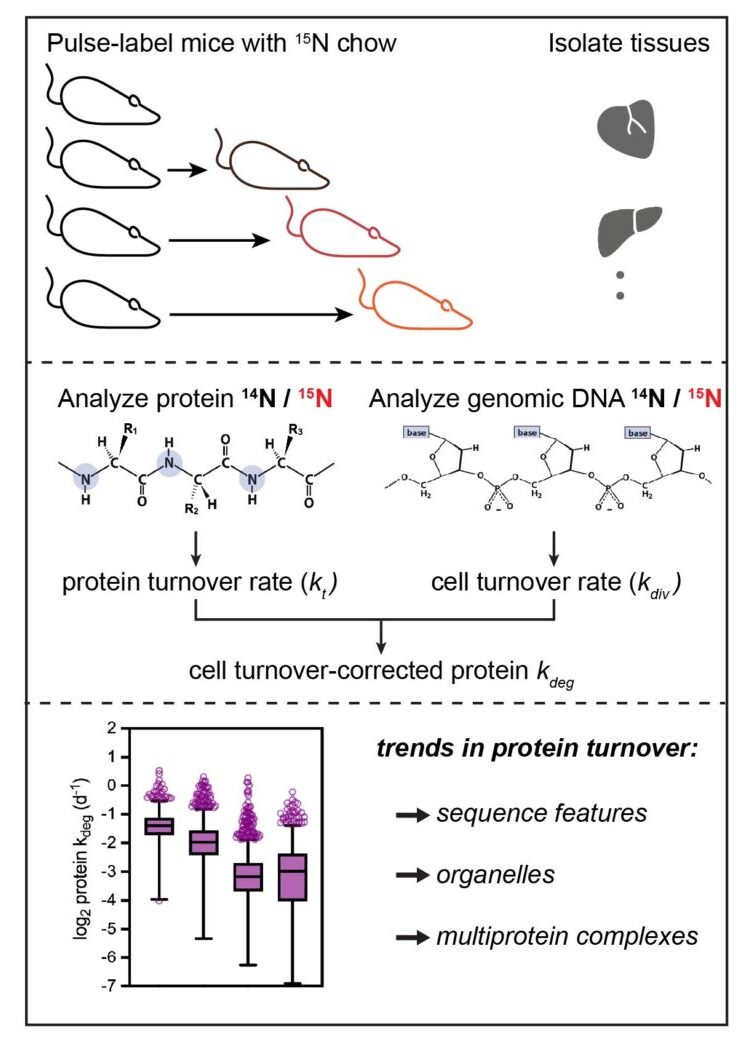
We became curious about how the lifetimes of nuclear proteins, including the lamins, are affected by cellular and tissue context.
In collaboration with the Ghaemmaghami lab at the University of Rochester, we developed a new approach to quantify protein turnover rates and cell proliferation rates within mouse tissues. We call this approach turnover and replication analysis by isotope labeling, or TRAIL.
This new approach revealed surprising features of global proteostasis and allowed us to probe the influence of laminopathy mutations on lamin stability specifically and nuclear proteostasis more broadly.
See publication here.